uKnee

Overview
uKnee is the first in vitro miniaturized model of a human osteoarthritic (OA) cartilage on chip, developed within uBeat® Compress Platforms. Healthy cartilage 3D microtissues are generated by culturing primary human articular chondrocytes embedded in an hydrogel in static condition for two weeks. Thanks to uBeat®, a mechanical overload triggers in few days the onset of OA pathological traits in the healthy cartilage, generating OA-like microtissues with a phenotype and genotype correlating with OA clinical evidences.
uKnee provides measurement of the key indicators of OA, including anabolic-catabolic balance changes, occurrence of inflammation, production of matrix degrading enzymes and changes in key cartilage molecular pathways.
Animal models and alternative in vitro platforms are unable to mimic the human pathology to this extent. uKnee can thus be used to test the efficiency of potential new disease-modifying osteoarthritis drug (DMOAD) , being the first in vitro platform able to replicate OA disease in vitro.
Model Characterization
Healthy human cartilage markers
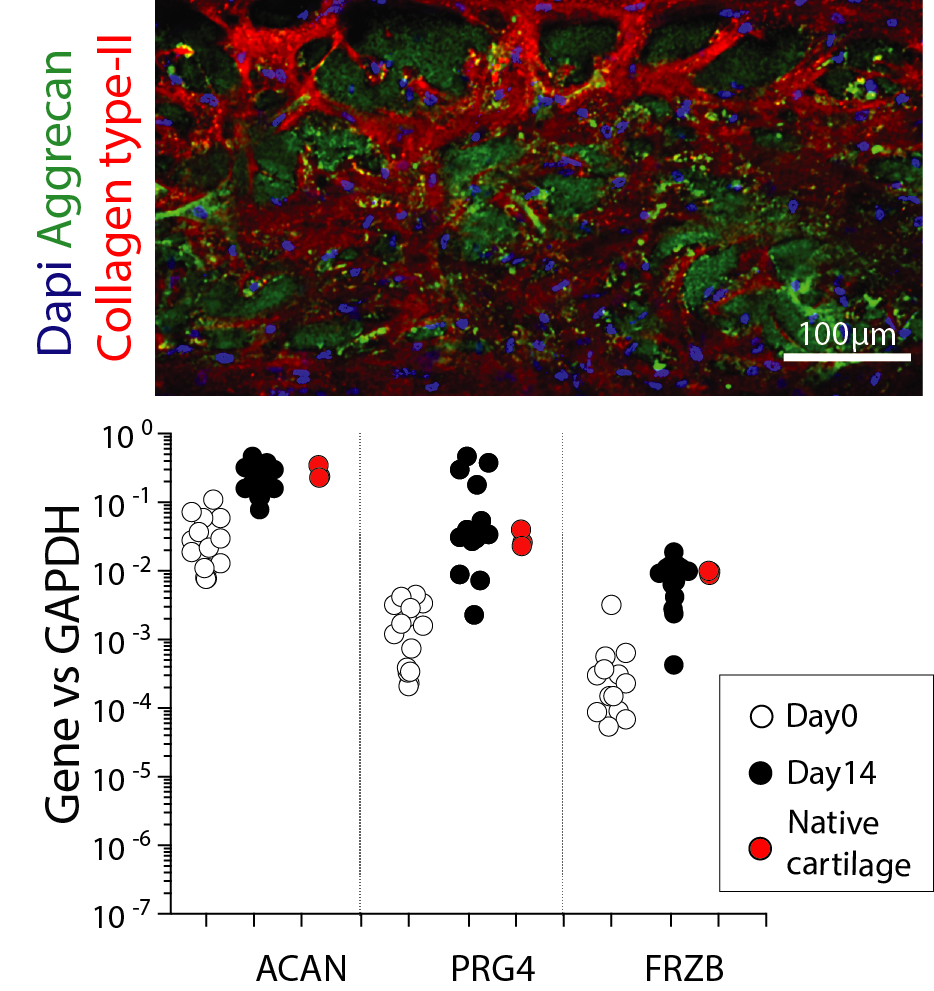
By culturing human healthy articular chondrocytes in static condition for two weeks within uBeat® Compress, a cartilage microtissue exhibiting a native-like deposition of extracellular matrix (abundant in collagen type-II and aggrecan) is obtained. Cartilage microtissues also achieved a characteristic gene profile matching those of human native cartilage.
Functionality
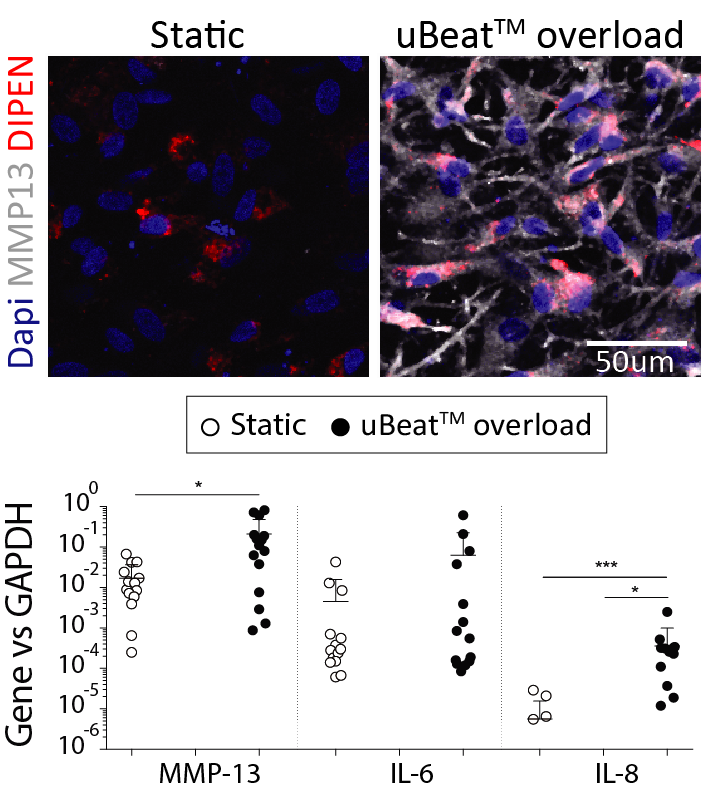
Onset of OA is correlated in vivo to mechanical damages. Accordingly, application of a uBeat® mechanical overload (i.e. hyperphysiological compression) induces a shift in cartilage homeostasis towards catabolism, as evidenced by loss of expression of anabolic genes (COL2A1 and ACAN), increased production of MMP13 cartilage degrading enzymes and up-regulation of inflammation related genes (IL6 and IL8).
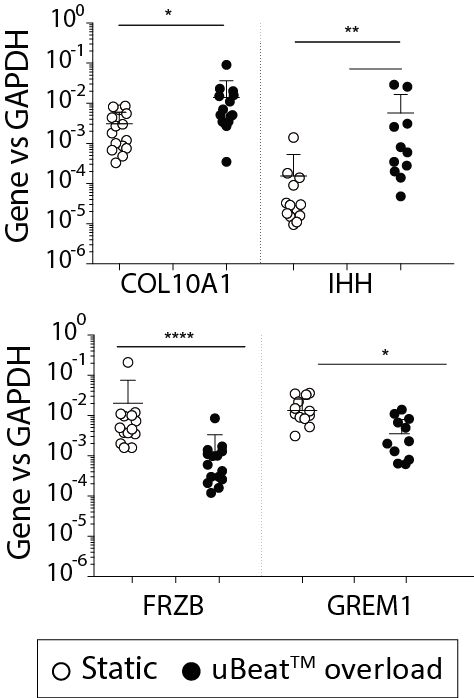
uBeat® pathological stimulation triggers the acquisition of a gene expression profile compatible with clinical evidences of OA. COL10A1 and IHH expression is upregulated, suggesting the triggering of a hypertrophic differentiation of the model towards transient calcified cartilage. GREM1, FRZB and DKK1 (BMP and Wnt signalling antagonists inversely correlated with OA onset) result downregulated, with levels matching those detected in native OA cartilage samples
Drug responses – validation for lead candidate efficacy
DMOAD compounds efficacy
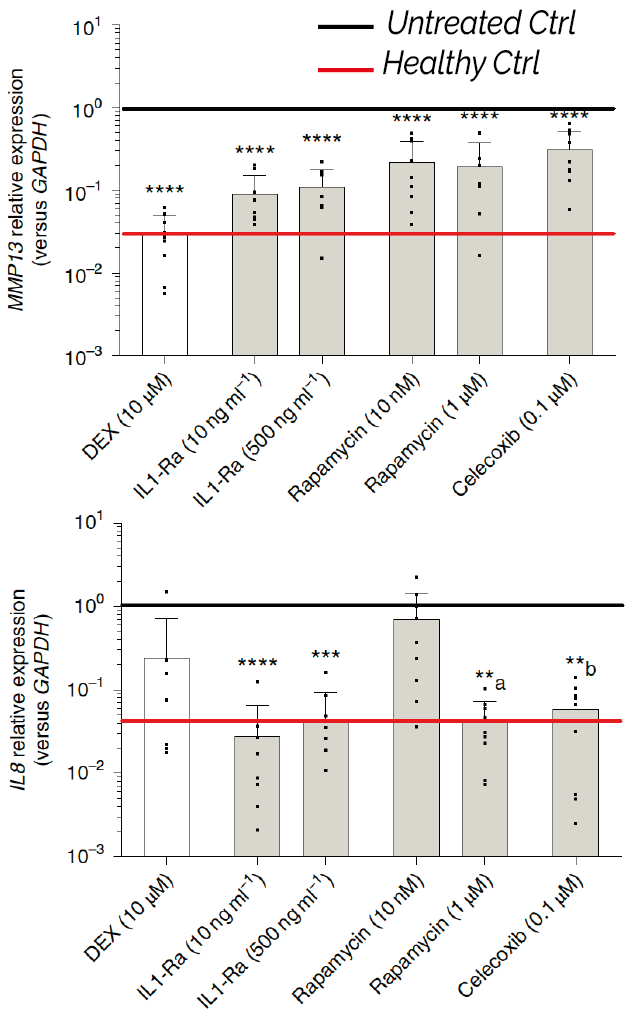
uKnee has been qualified for assessing anti-inflammatory and anticatabolic responses of both commercially available drug and DMOAD compounds currently under investigation. When administered to OA-microtissues, the drugs downregulated the expression of peculiar genes (e.g. MMP13, IL8), in accordance with the mode of action of the tested candidates, restoring the healthy cartilage levels. Of note, in the case of rapamycin, uKnee was able to predict effects not detectable by a traditional ‘cytokine-based’ model, highlighting its advantage compared to commonly used simplistic in vitro assays for DMOAD efficacy studies.
Check out our publications.
Injectable therapeutics efficacy
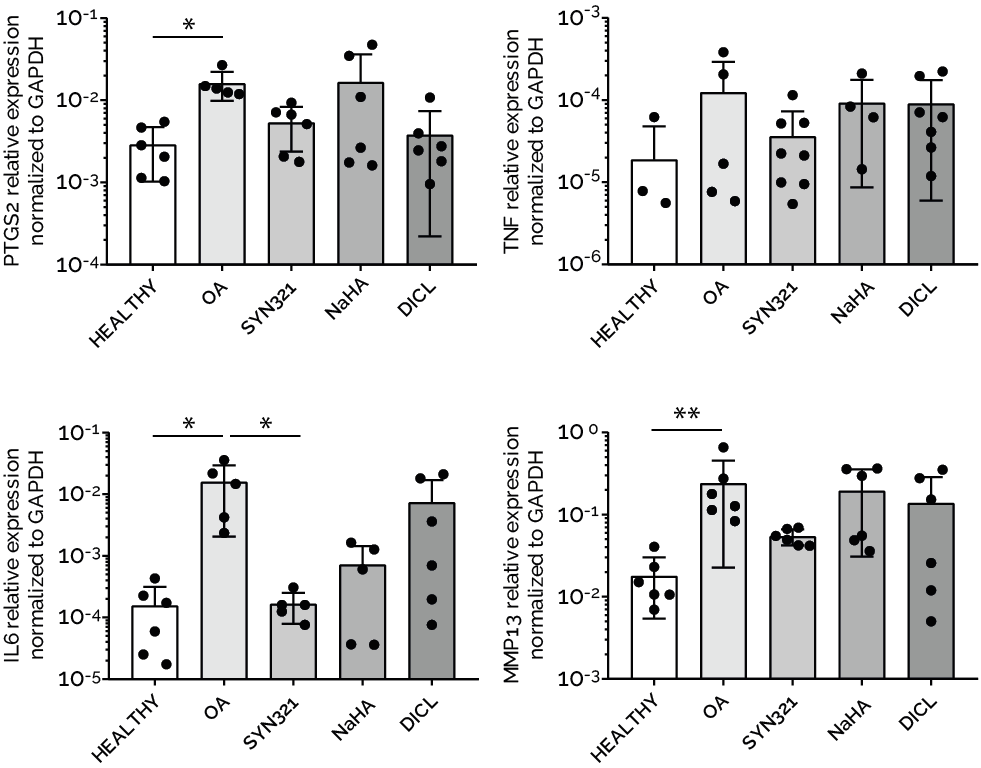
An innovative configuration of uKnee has been specifically developed and qualified for studying the efficacy of an injectable therapeutics for treating OA. The product is currently under development from our sponsor Synartro, a Sweden biotech company developing a new anti-OA combined injectable therapy. The results indicated that the product downregulated inflammatory markers (i.e. PTGS2, TNF, IL6) and reduced the matrix degradation of OA cartilage microtissues (i.e.MMP13, COL1A1, ACAN). The data on the therapeutic efficacy and mode of action acquired from uKnee have been used by Synartro to file an IND application, to compliment data from animal models.
Check out our publications.
Multi-tissue joint models
uKnee is currently applied in collaboration with internationally recognized opinion leaders in the orthopedics field (Galeazzi Orthopaedics Institute, University Hospital Basel) to develop multi-tissue model of the joint. Cartilage-synovium cross-talk, osteochondral compartment development and immune compartment influence on the OA pathology represents some of the fields currently under investigation. Application beyond OA are also currently being developed in collaboration with Politecnico di Milano, with a first proof of concept being released on rheumatoid arthritis.
Key Advantages
- Human based model
- Contains relevant cell populations
- Versatility to perform drugs, injectable therapeutics or advanced therapies efficacy studies.
- Replication of OA clinically relevant biomarkers
- Clinically relevant pharmacological responses