BiomimX Applications - Safety and Toxicology
Predict safety and toxicity of therapeutic candidates in human models
The Challenge
Obtaining clinically relevant data on candidate toxicity from pre-clinical models.
The risk to introduce ineffective or even toxic molecules in clinical trials is still high. Despite passing preclinical safety screenings in animals, about 30% of drugs fail during human trials due to toxicity. Currently used preclinical tests indeed are only an imperfect representation of the human system, providing unreliable data to effectively guide a safe preclinical-to-clinical transition.
BiomimX's Solution
Anticipate human responses during the preclinical phase to ensure seamless data translation into clinical trials.
BiomimX’s uBeat® powered models offer the possibility to assess drug candidate toxicity on human tissue replica, enabling to early predict the clinical outcome. Our technology can lead to progress safer drug candidates and to reduce the need for animal testing, finally boosting your R&D performances.
Cardiac Safety
Screen your therapeutic candidates’ cardiotoxicity in uHeart model by investigating drug-induced changes in electrophysiology, contractility and calcium handling parameters.
uHeart is a 3D model of physiological human beating myocardium suitable to detect therapeutic candidates’ cardiotoxicity as early as possible in the drug development pipeline. uHeart has been validated in collaboration with with 12 reference compounds to detect field potential prolongation and arrhythmic events (83.3% sensitivity, 100% specificity and 91.6% accuracy) and with 23 compounds affecting contractility and calcium handling.
Check out our publications (Marsano et al, LabOnChip, 2016; Visone, Lozano Tox Sci, 2022) and posters (ESTIV2024, SPS2024 and EUROTOX2024).
Intestinal Toxicity
Screen your therapeutic candidates’ intestinal toxicity on uGut model by investigating intestinal barrier inflammation and integrity.
uGut is a 3D model of the gastrointestinal tract suitable to detect therapeutic candidates’ toxicity as early as possible in the drug development pipeline. uGut shows faithful responses to inflammatory stimuli, as evidenced by the impairment of intestinal barrier functionality and by the activation of pro-inflammatory pathways. uGut is the ideal platform to set-up drug candidates’ absorption assays in ADME studies.
Check out our publications (Ballerini et al, Nature biomed Eng, 2024) and posters (Ballerini et al, EUROocs 2024; Karol Kugiejko).
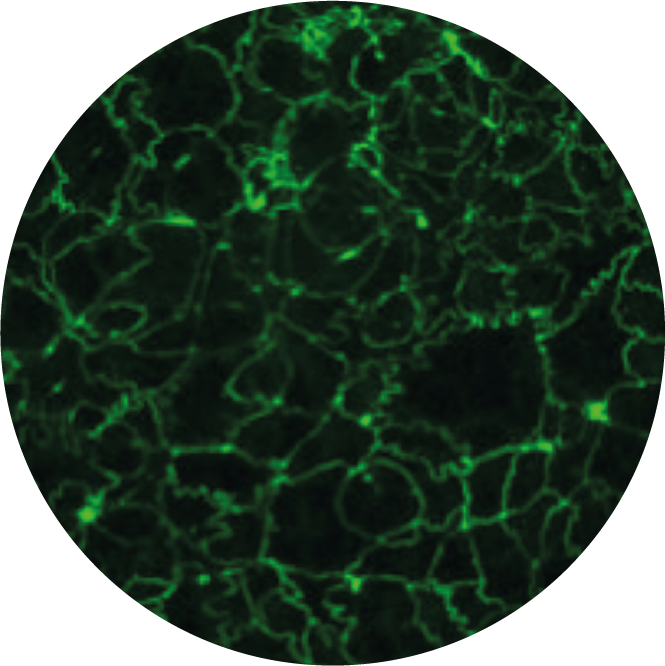
Liver Safety
Screen your therapeutic candidates’ hepatotoxicity on uHep model by investigating drug-induced liver damages and changes in functionality.
uHep is a 3D model of the liver, suitable to detect therapeutic candidates’ hepatotoxicity as early as possible in the drug development pipeline. uHep represents a liver sinusoid-on-chip system which may account for vasculariziation. uHep shows high viability and functionality, as demonstrated by low level of LDH and high level of albumin and cytochrome P450 activity. uHep is the ideal platform to assess hepatic metabolism of drug candidates’ in ADME studies.
Check out our publications (Ferrari et al., Biomedical Materials, 2022; Ferrari et al., Biomicrofluidics,2023).

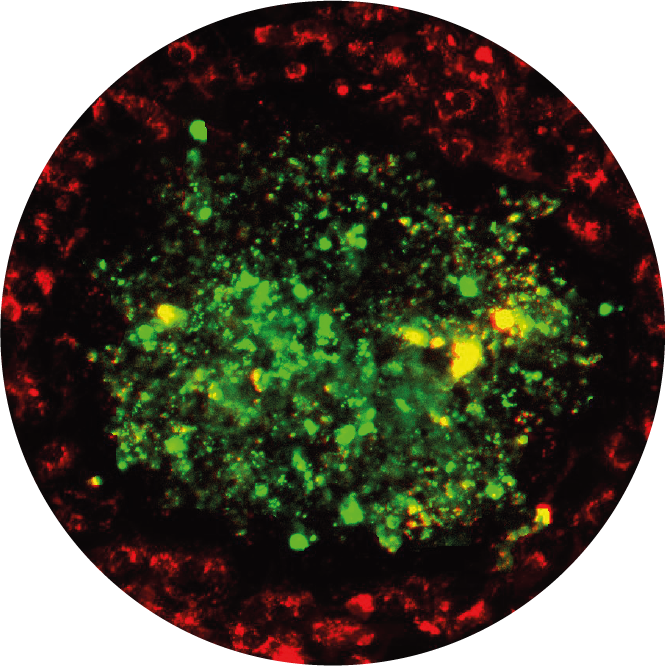
First-Pass Cardiotoxicity
LivHeart is a multi-organ on chip encompassing a metabolic competent liver and the 3D uHeart, suitable to detect cardiotoxicity after hepatic metabolism. LivHeart effectively predicts functional and structural cardiotoxicity of Terfenadine (and its hepatic metabolites Fexofenadine). LivHeart represents a promising model for more representative therapeutic candidates’ cardiotoxicity studies and metabolism investigations for ADME assessment.
Check out our publications (Ferrari et al., Adv Health Mat, 2023).
Take Advantage from our uBeat® based solution
- Screen toxicity of pre-clinical drug candidates in human-based set-up.
- Detect toxicity of compounds to compare and contrast preclinical animal studies.
- Uncover toxicity mechanisms in candidates with unexpected clinical outcomes.
