uGut

Overview
uGut is a miniaturized model of functional gastrointestinal track on chip developed within uBeat® Stretch Platforms.
Thanks to uBeat®, a mechanical stimulation resembling the intestinal peristalsis is applied to human relevant gut cell populations (i.e. epithelial and endothelial cells) cultured in a 3D environment. Within few days, the generation of functional human epithelial and endothelial barriers is achieved.
uGut can provide measurement of the key function of the intestine to understand changes in barriers viability and functionality. uGut results the ideal platform to screen drug toxicity, to perform drug absorption studies and to understand cell-microbiota interaction.
Model Characterization
Epithelial and endothelial barriers formation
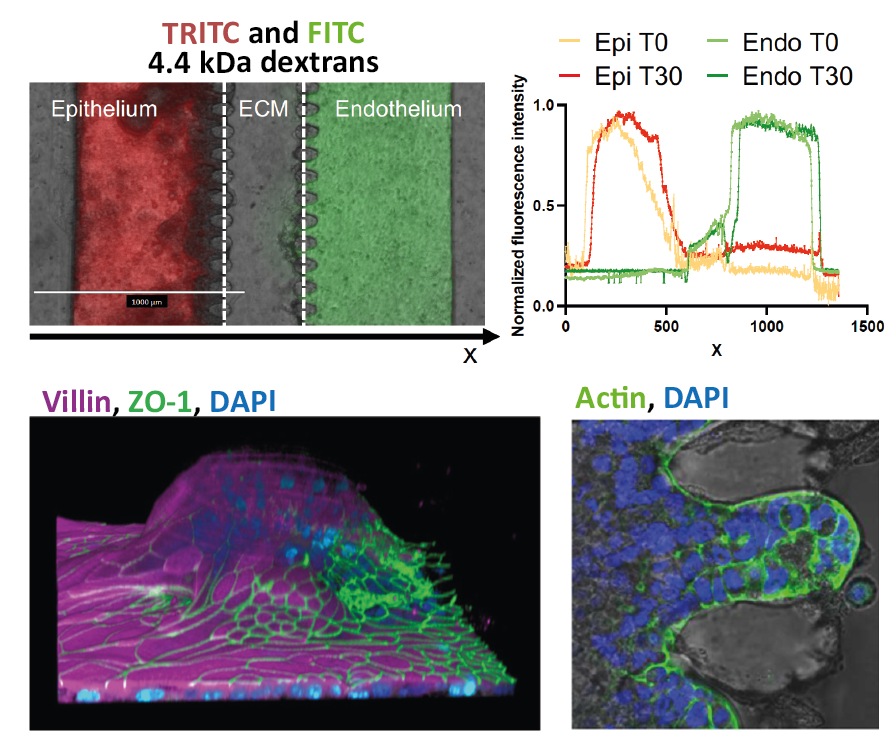
uGut features microchannels lined with living human intestinal epithelium over an hydrogel-based ECM-like structure. Peristalsis-like mechanical deformation provided by uBeat® enhances the formation of intestinal villus 3D microarchitecture and functional barriers (i.e. dextran retention by the model), and promotes intestinal cell proliferation, maturation, and mucus production. uGut has been interfaced with endothelium and co-cultured with other cell types (e.g., immune cells) and gut microorganisms (microbiome).
Drug responses – validation for lead candidate toxicity
Barrier impairment upon pro-inflammatory stimulation
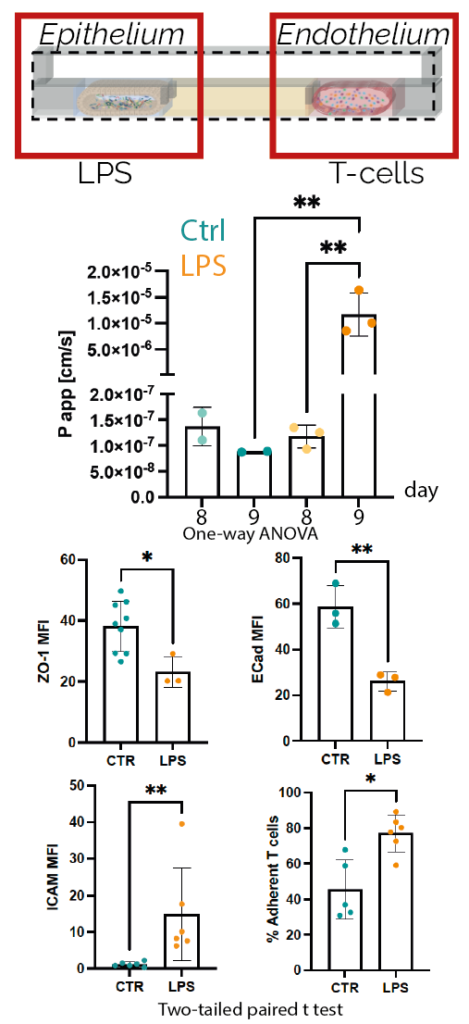
uGut has been qualified in collaboration with the Istituto Europeo di Oncologia (IEO) for assessing the toxic effects of pro-inflammatory stimulations on functional epithelial barriers. Specifically, Lipopolysaccharide (LPS) administration to functional and tight barriers, evidenced a loss of epithelial barrier integrity (i.e. decreased ZO-1 and E-cad epithelial markers, impairment of dextran retention) and an endothelial cells activation (i.e. increased ACAM and % of adherent T-cells) with a concurrent upregulation of inflammatory pathways such as IL-6, TNF, and VEGF.
Advanced therapeutic screening – validation for microbiome interaction
Microbiome therapy efficacy
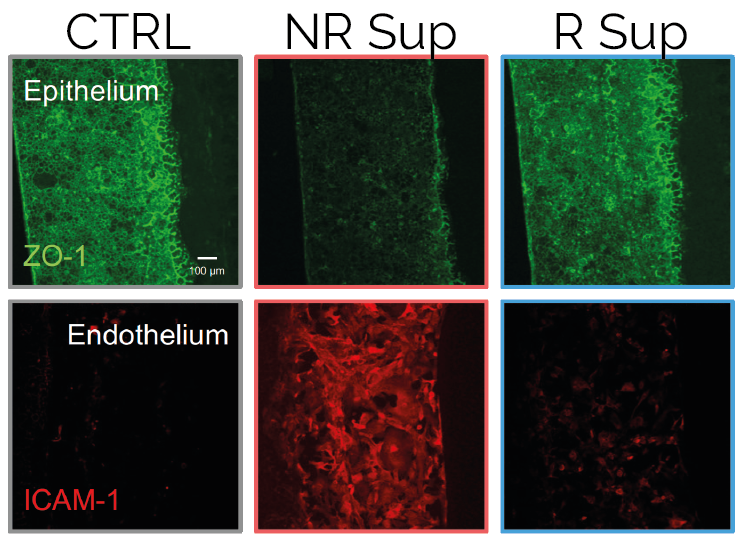
uGut has been used in collaboration with the Istituto Europeo di Oncologia (IEO) to predict clinical outcomes of an anti-cancer Immune checkpoint inhibitors (ICI) therapy through fecal microbiome transplantation. uGut was demonstrated to respond to microbiome from non-responder melanoma patients (NR) by losing barrier integrity, while microbiome from responder melanoma patients was found to not disturb the intestinal epithelium’s normal architecture or functionality. Microbiome culture was demonstrated to impact also the vascular endothelial compartment, with NR microbiome causing loss of vascular barrier integrity and endothelial cell activation. These data highlight the suitability of uGut to reliably predict clinical outcomes of ICI therapy through fecal microbiome testing, towards patient-specific applications.
Key Advantages
- Human based model
- Contains different relevant cell populations
- Physiologically relevant mechanical stimulation
- Clinically relevant therapeutics responses towards patient-specific applications