uScar

Overview
uScar is a miniaturized in vitro model of a fibrotic human heart, developed in a chemical-free setup within uBeat® Stretch Platforms.
Thanks to uBeat®, a mechanical training phase resembling the heart beating is applied to human cardiac fibroblasts cultured in 3D, triggering the onset of key fibrotic traits.
uScar provides measurement of activation/downregulation of inflammatory, mechano-related genes, cell fibrotic phenotype switch and matrix deposition.
uScar is able to respond to anti-fibrotic drugs, enabling for the first time to perform unbiased screening of TGFb-related targets, and to assess the capabilities of new anti-fibrosis advanced therapeutics in preventing or reverting the pathology, in a mechanically relevant environment.
Model Characterization
Mechano-related pathway and fibrotic traits induction
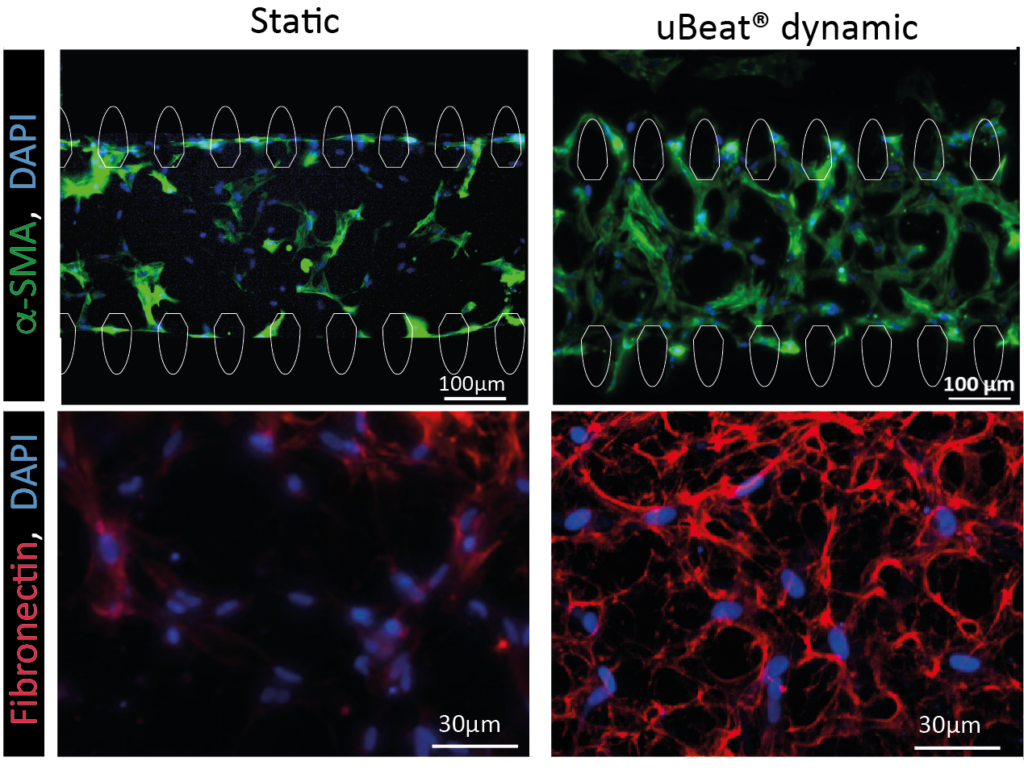
The sole provision of a uBeat® cyclic beating-like mechanical stimulation (i.e. 10% uniaxial strain at 1 Hz) applied to cardiac fibroblasts induces fibrotic traits in miniaturized human 3D microtissues. Mechanosensing-dependent or inflammation-related pathways (e.g. Roha-Rock, Hippo, WNT/𝛽-catenin) results activated after 7 days of mechanical stimulation. Moreover, cyclic mechanical strain resulted as the principal trigger for the induction of fibroblast-to-myofibroblast transition and for the enhanced matrix deposition, rich in collagen, fibronectin and aggrecan. These results corroborated the suitability of uScar to generate a representative human cardiac fibrotic model without the use of exogenous cytokines, which may create a bias affecting the screening results of potential anti-fibrotic drug candidates acting on the same pathway.
Drug responses – validation for therapeutic candidate efficacy
Anti-fibrotic drug efficacy

uScar has been qualified for assessing anti-fibrotic effect of a clinically used drugs modulating the TGFb pathway (i.e. Tranilast) and of a medication used for the treatment of idiopathic pulmonary fibrosis (i.e. Pirfenidone). When administered to the culture since the beginning the drugs prevented the uBeat®-induced onset of fibrotic traits in the microtissues, limiting the cell-phenotype transition and reducing the deposition of matrix components, restoring values similar to the healthy microtissues.
The capability of uScar model to predict anti-fibrotic effect of small molecules not only targeting pathways of cardiac fibrosis, but also of other type of fibrosis highlighted the possibility to exploit our human set-up also for drug repurposing.
Advanced gene therapy efficacy
uScar has been used in collaboration with Politecnico di Torino, to test the efficacy of an innovative gene therapy against infarction, specifically designed to directly reprogram in vivo cardiac fibroblast into cardiomyocytes. While this therapy showed promising data in standard 2D cell culture models, the introduction of a native-like mechanically active environment (matching the situ of injection) with uBeat® highlighted some limitations in the efficacy of the therapy. Specifically, uBeat® prevented the CM-like phenotype switch of fibroblast previously transfected in 2D, promoting the formation of fibrotic-like microtissues. Moreover, the therapy was found to be only mildly able to mitigate the fibrotic traits acquired in uScar microtissues developed under dynamic culture condition and ineffective in inducing direct cell reprogramming. These data highlight the importance of incorporating in vivo like 3D stimulations into in vitro models, to better anticipate a failure of the therapy that could have costs time and money in clinical trials.
Immunocompetent cardiac fibrosis model
uScar is currently being exploited in the context of an EU project, MIRACLE, which aims at uncovering the intricate layers of inflammatory regulation in cardiometabolic disease. The model will recapitulate the diverse features of cardiac fibrosis and will elucidate the contribution of different cells (eg, cardiac fibroblasts, cardiomyocytes, monocytes and macrophages) into the pathology onset and progression. This technology will offer a high-throughput tool to identify new targets and to investigate the efficacy of anti-fibrotic drugs or advanced therapies in preventing or reverting heart failure.
Key Advantages
- Human based model
- Contains different relevant cell populations and stimulations
- Relevant cardiac fibrosis markers
- Clinically relevant responses to therapeutics

