Advance new therapies
Stay human
BiomimX unlocks in vitro models reflecting human complexity, towards the design of effective treatments tailored for patients.
Beating Organs-on-Chip are the next generation of pre-clinical models: human, dynamic, 3D.
BiomimX unlocks in vitro models reflecting human complexity, towards the design of effective treatments tailored for patients.
Beating Organs-on-Chip are the next generation of pre-clinical models:
human, dynamic, 3D.
Advance new therapies
Stay human
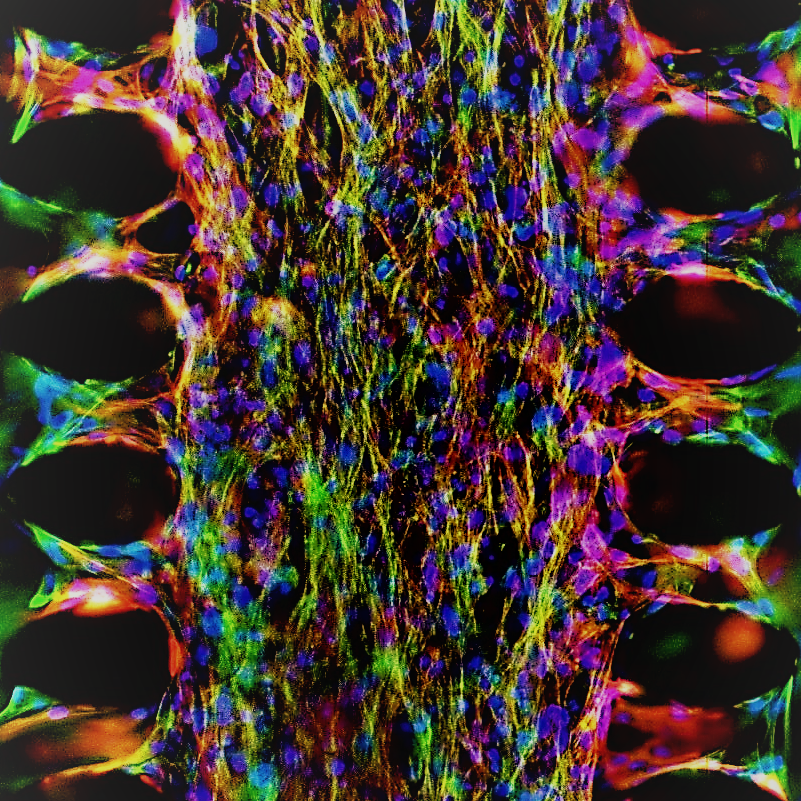
Discover the therapies of tomorrow
by catching human data today
Every tissue in the body is subjected to motion.
Our Beating Organs-on-Chip finally bring this motion into the
3D native microenvironment,
offering clinically relevant human in vitro setups.
Every tissue in the body is subjected to motion.
Our Beating Organs-on-Chip finally bring this motion into the 3D native microenvironment,
offering clinically relevant human in vitro setups.
Discover our Services
Test Your Compounds in Human Relevant in vitro Models
Boost Your Pipeline
BiomimX provides services to enhance every step of your pipeline by relying on native-like data from uBeat®-powered models.
Partner with us and leverage on BiomimX®’s expertise and strong scientific know-how, to get your product profiled.
Get inspired by our Applications
Advanced Therapies
Advanced Therapies
Predict the toxicity and efficacy of gene, antibody and cell therapeutics in human models.
Microbiome interaction
Elucidate gastrointestinal microbiome interaction at patient specific level.
Discover our Products
Develop Your in vitro Physiological & Pathological Models
uBeat® Platform & uBoX Pro
The uBeat® Setup supports the culture of 3D microtissue models within the uBeat® Platform.
The patented uBeat® Technology allows to provide a controlled mechanical stimulation to the microtissues through the uBoX Pro.
The mechanical stimulation will drive the model development, mimicking the native human tissues.
Get inspired by our uBeat®-powered models
uHeart
uKnee
uGut
uHeart
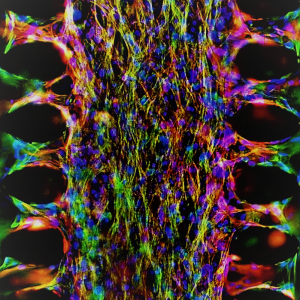

A functional beating human Heart-on-a-Chip with real time electrical activity recording functionality.
uKnee
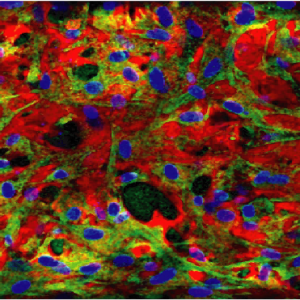
The first in vitro miniaturized models of human healthy and osteoarthritic Cartilage-on-Chip.
uGut
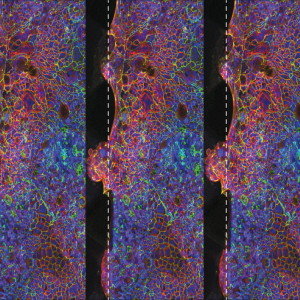

A human model of the gastrointestinal tract, with intestinal epithelium and endothelium.
Learn more about our
Biological Models and Scientific Expertise
From our high-ranked publications on peer-reviewed journals

An Advanced Mechanically Active Osteoarthritis-on-Chip Model to Test Injectable Therapeutic Formulations: The SYN321 Case Study
Advanced Healthcare Materials
Sep 2024

In Vitro Mechanical Stimulation to Reproduce the Pathological Hallmarks of Human Cardiac Fibrosis on a Beating Chip and Predict The Efficacy of Drugs and Advanced Therapies
Advanced Healthcare Materials
Nov 2023

LivHeart: A Multi Organ-on-Chip Platform to Study Off-Target Cardiotoxicity of Drugs Upon Liver Metabolism
Advanced Materials Technologies
Jan 2023
Predicting human cardiac QT alterations and pro-arrhythmic effects of compounds with a 3D beating heart-on-chip platform
Toxicological Sciences
Oct 2022
Read all Publications
Meet us

February 11-13 2025
Join our Commercial Science Leader, Dr. Erika Ferrari and our Business Developer Victoria Rabsiun at the WORD+ 2025 in Cambridge, UK.

Latest News
🎉 Our Mechanically Active Gut-on-Chip Model is finally out on Nature Biomedical Engineering!
This work definitely represents a #Milestone in BiomimX® Srl scientific and technological achievements and we believe that it provides the joining link between in vitro models and personalized therapies. This opens a new era in the Organs on Chip field and in drug discovery and development enabling groundbreaking insights into microbiome interactions and absorption studies.
Our uGut model is the first Gut-on-Chip (GoC) in literature to replicate human intestinal architecture and functionality, incorporating both faecal microbiome and peristaltic-like movements, thanks to our patented uBeat® Technology. Remarkably, uGut has demonstrated its ability to:
✅ Distinguish between microbiomes of melanoma patients responding vs non-responding to immunotherapies.
✅ Identify epithelium-specific biomarkers and microbial factors linked to clinical outcomes.
A huge thank you to everyone who made this achievement possible! Special thanks to Luigi Nezi’s group at IEO Istituto Europeo di Oncologia, our CTO and Co-founder Marco Rasponi’s team at MiMicLab, and Mattia Ballerini for his incredible work across both institutions.
Get in touch with BiomimX Team to develop collaborations around uGut!
Cooperation, sharing and synergy, three key-words that marked the beginning of PHOENIX project, funded under the Horizon Europe programme (HORIZON-HLTH-2024-TOOL-05-two-stage) and coordinated by Prof. Marco Rasponi from the Department of Electronics Information and Bioengineering of Politecnico di Milano.
PHOENIX aims to revolutionize biomedical research by developing advanced Organs-on-Chip (OoC) platforms that will foster the investigation of complex pathologies, focusing on cardiac and neuro-muscular diseases. Such platforms will enhance our understanding of disease mechanisms, accelerating drug discovery and ultimately leading to reduced animal testing.
On February 3-4, 2025, the kick-off meeting brought together the partners of the project, nine organizations coming from various parts of Europe, each of them bringing diverse background and expertise to the table.
Milestones were set, the roadmap was outlined, now the project is ready to start. Stay tuned!
We are incredibly proud to announce that Queen Mary University of London recently acquired different in vitro model facilities for educational purposes and BiomimX uBeat® setup, is one of them!
These facilities have been funded through a mixture of commercial partnerships, internal funding, research project grants and a large infrastructure grant from the NC3Rs and BBSRC. The facilities are integrated with category II cell culture, advanced microscopy and molecular biology and biochemistry facilities.